Breast Cancer Genetics, Genomics, and Diagnostic Tools: What Patients Need to Know
Presentation by Dr Radhika Acharya-Leon, University of Colorado
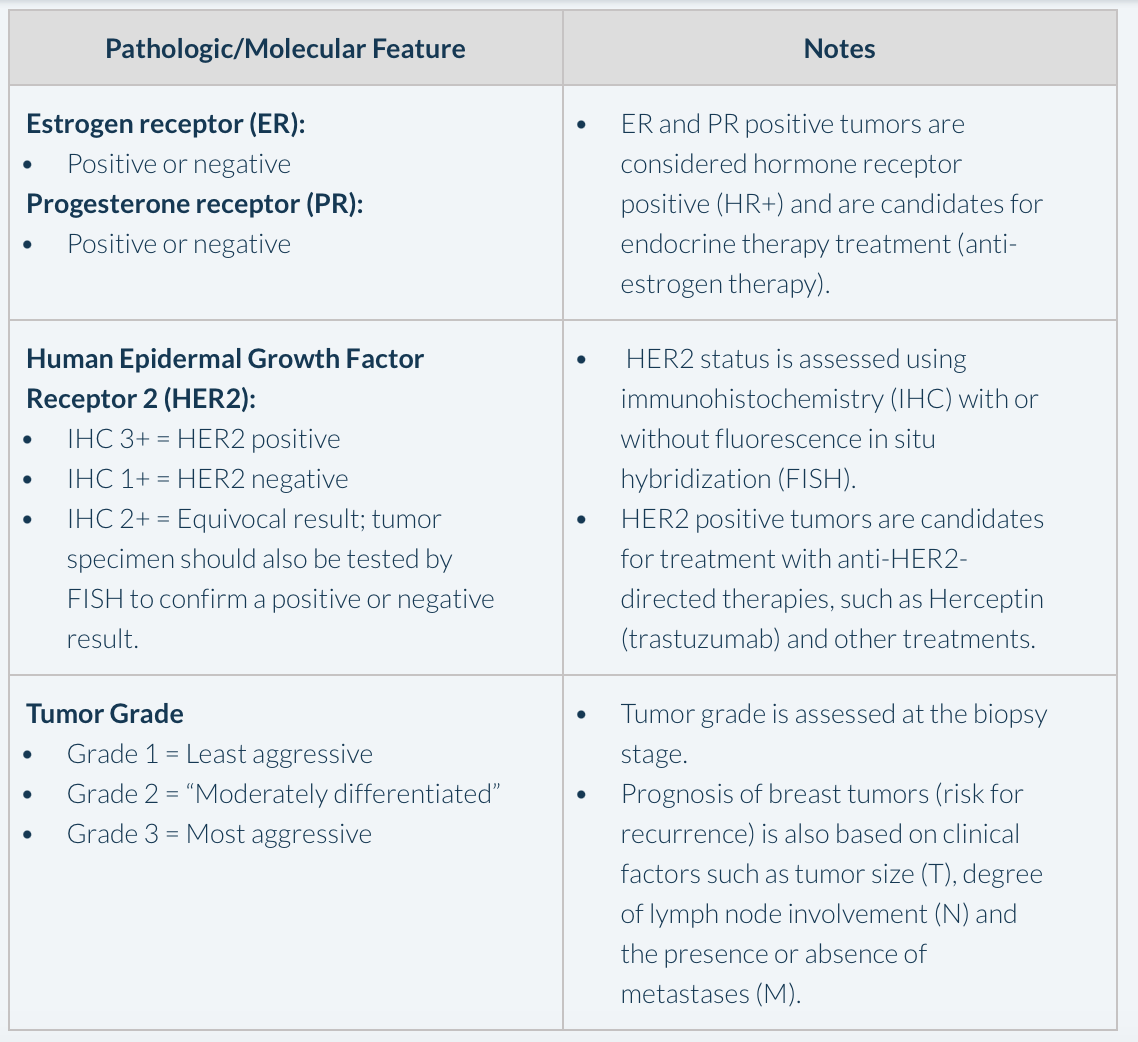
At the 2023 Meeting of the Oncology Nursing Society Metro Denver Chapter (MDONS) Dr Radhika Acharya-Leon, DO, from The University of Colorado provided an overview of some of the most important types of genetic testing and diagnostic tools currently used in the setting of early breast cancer, using a case-based approach. She described the case of a 41-year-old premenopausal woman with a palpable mass in her breast that is confirmed using a diagnostic mammography and ultrasound. On biopsy, the tumor is found to be an invasive ductal carcinoma (one of the most common types of breast cancer) that is 5.1 centimeters, grade 2 (on a scale of 1 to 3) and the tumor is both estrogen receptor (ER) and progesterone receptor (PR) positive (i.e., hormone receptor positive, or HR+), and human epidermal growth factor receptor 2 (HER2) negative. Dr Acharya-Leon explained that because the patient’s tumor is HR+, endocrine therapy (i.e., anti-estrogen therapy) will be at least one component of her treatment plan, with the potential to reduce her risk of her breast cancer recurring by about 50%. The key features to be assessed with an early breast cancer diagnosis are outlined in Box 1.
Box 1. Key Pathologic and Molecular Features to be Assessed in Early Breast Cancer
Genetic Testing
Dr Acharya-Leon described some of the relevant clinical aspects of the case that strongly suggest a need to do genetic testing in this patient, specifically because she is relatively young, pre-menopausal, and she also had a relevant family history of breast cancer, pancreatic cancer, and colon cancer. She noted the importance of discussing the risks, benefits, and limitations of genetic testing, as it can cause some anxiety for the patient to know whether they have an increased risk of certain cancers, based on their genetics. She further noted that, in addition to the well-known genes BRCA1 and BRCA2 that can lead to an increased risk of breast cancer, mutations in other genes such as CHEK2, ATM, and PALB2 may also have an impact on familial breast cancer risk. In this case, genetic testing using an 84-gene testing panel was completed and the patient was found to have a mutation in the BRCA2 gene as well as another mutation of unknown relevance for cancer, also called a variant of unknown significance, or a VUS. Importantly, she stressed that a VUS is not considered a “positive”, or actionable, genetic testing result. Because of her inherited BRCA2 mutation, the patient has an annualized risk of 2.6% for developing a second cancer in the opposite (contralateral) breast, corresponding to a 21% risk for a contralateral breast cancer over a ten-year period. Dr Acharya-Leon also noted her risk for ovarian cancer was also increased by virtue of her BRCA2 mutation.
Adjuvant Therapy: Tumor Genomic Classifiers
Continuing with the case, the patient elects to undergo a bilateral mastectomy, due to her being positive for a BRCA2 mutation. The pathology results following surgery stage her tumor as T3/N0/M0, meaning that her tumor (T) is relatively large, at 5.1 cm, her lymph nodes are negative for cancer (N0), and she has no evidence of metastases, or spread of the tumor to distant sites (M0). As noted above, her initial biopsy results indicated that her tumor was HR+, and therefore she is a candidate for endocrine therapy. At this point, additional genomic testing on the tumor tissue using a 21-gene “genomic classifier” was performed, to determine her overall risk of a distant cancer recurrence over the next ten years, and to determine the overall benefit that chemotherapy would have in reducing this recurrence risk (Box 2). The results of this testing came back as genomic “high risk”, and indicate that she has a 20% risk for a distant recurrence if she were to complete a full course (5 years) of adjuvant endocrine therapy. Importantly, if she were to forgo endocrine therapy, her recurrence risk in this setting would essentially double, to 40%. The results also indicate that chemotherapy would be beneficial to use in this patient to further reduce her recurrence risk.
Dr Acharya-Leon noted that the genomic classifier used in the case (21-gene test) is one of the most validated tests and the most widely used, as it provides both prognostic information (defining the overall risk of recurrence over 10 years) as well as predictive information (defining the overall benefit of using chemotherapy as a means to reduce this recurrence risk). She noted that the test used in this case is validated only for patients who have HR+ and HER2 negative breast cancer, and the test can also be used in patients with (unlike the example) with between 1 and 3 positive lymph nodes. Dr Dr Acharya-Leon also noted that other genomic classifier tests are available (e.g., a 70-gene test), and are approved to determine overall recurrence risk, and in some cases the benefit of chemotherapy, but have not been as widely used in the United States.
Box 2. About Genomic Classifiers in Early Breast Cancer
Fertility, Chemotherapy, and Endocrine Therapy
Continuing with the case, Dr Acharya-Leon noted that, before deciding to proceed with chemotherapy, the patient reviewed her options for fertility, as she had two children at present but had been considering a third. She noted these are important conversations to have with the patients in this position, since chemotherapy can cause a permanent menopause that would make future childbearing impossible. As such, the patient was referred to onco-fertility for evaluation, but ultimately decided not to proceed (with a further pregnancy), and initiated chemotherapy. After completing her treatment, she underwent a bilateral oophorectomy (removal of both ovaries) since she was BRCA2 positive and at increased ovarian cancer risk. She also initiated endocrine therapy with an aromatase inhibitor (AI), and Dr Acharya-Leon explained that this is needed, since her tumor is HR+ and by definition hormone-responsive, and even though her ovaries had been removed (and are no longer producing estrogen) it is still possible to convert “pre-estrogens” in the body to estrogen, and as such, endocrine therapy is still indicated for this patient. The patient is also tested for her baseline bone density (using a ‘DEXA’ scan) and is started on a bisphosphonate treatment to protect her bones, as loss of bone density is a common side effect of AI therapy. Bisphosphonate therapy is also known to reduce breast cancer recurrence risk in patients on AIs.
Circulating tumor DNA (ctDNA)
Circulating tumor DNA, or ctDNA, is a new methodology that allows for detection of residual tumor in the body, also called “minimal residual disease” or MRD (Box 3). Dr Acharya-Leon noted that this patient was monitored for disease recurrence using ctDNA testing, which is capable of detecting recurrence before it would be detected using a conventional CT scan or other imaging methods. Because ctDNA testing can detect a recurrence before it becomes detectable by imaging, it can be a source of significant anxiety for the patient, as those individuals with detectable ctDNA in their blood after surgery are more likely to recur (and therefore have a much poorer prognosis) than those who do not. Conversely, those with negative ctDNA after surgery can be more reassured that their cancer will likely not recur. Dr Acharya-Leon noted that the overall clinical utility of ctDNA testing in breast cancer is not yet fully resolved at present; this is mainly due to the potential for a positive result to cause patient anxiety and the fact that it is not yet known that intervening on the basis of a positive ctDNA result alone will make a difference in patient survival.
Box 3. About ctDNA Testing
Circulating tumor DNA (ctDNA) testing can be used to detect the presence of residual cancer cells in the body (called “minimal residual disease” or MRD), that have the potential to cause a cancer recurrence later on.
Testing for ctDNA is performed using a simple blood test, and the results can be either positive (ctDNA detected) or negative (ctDNA not detected):
Patients with ctDNA positive status following surgery have a poorer prognosis, and their cancer is likely to recur.
Patients with ctDNA negative status following surgery have a good prognosis, and their cancer is unlikely to recur.
Currently, the role of ctDNA testing in breast cancer is controversial, as a positive result can cause significant patient anxiety, and there are no established guidelines for ctDNA testing.
ctDNA testing can detect an impending cancer recurrence before it can be detected using imaging (e.g. CT scan).
It is not yet known whether treating a recurrence on the basis of a positive ctDNA result alone is beneficial for the patient.
Tumor Genetic Profiling in Metastatic Disease
Continuing the case, Dr Acharya-Leon noted that findings from subsequent CT scan of the chest, abdomen and pelvis was negative, however, a bone scan revealed numerous bone lesions. Upon biopsy of the bone lesions, they were found to be consistent with metastatic breast cancer (meaning her original breast cancer had spread to the bone). Pathology of the bone lesions, however, showed them to be ER and PR negative, as well as HER2 negative, indicating that the tumor had changed, and was no longer HR+ and responsive to endocrine therapy. In this regard, Dr Acharya-Leon noted that it is especially important to re-biopsy possible metastatic sites in order to prove that it is cancer, that it is the same cancer, and if so, what are the relevant biological characteristics of the cancer recurrence. In this case, the specimen was also sent for additional testing to look for specific molecular targets that can guide therapy in metastatic disease. For example, patients with overexpression of a molecule called PD-L1 may be candidates for a different type of treatment called immunotherapy, or if mutations are found in other genes such as ATM or PALB2, the patient could be a candidate for other targeted treatments. In this case, Dr Acharya-Leon noted that due to her previously identified mutation in BRCA2, the patient underwent therapy with Olaparib, an orally administered “PARP-inhibitor” type drug which has shown efficacy in breast cancer, specifically in patients who have BRCA1/2mutations. She concluded the case by noting that the patient had an initial good response to Olaparib, and continues to do well on the treatment.
Speaker Disclosure Information: Dr Acharya-Leon listed no disclosures for this presentation.
You can view Dr Acharya-Leon’s full presentation from the MDONS Conference here: